FWF P23317 - Antiproliferative and antimigratory activity of I3MO
| Project title: | Antiproliferative and antimigratory activity of I3MO. |
| Project number: | FWF stand alone project P23317 |
| Project leader: | Dr. Elke Heiß elke.heiss@univie.ac.at |
| Duration: | 2011 - 2013 |
| Location: | University of Vienna, Faculty of Life Sciences Department of Pharmacognosy Althanstraße 14, 1090 Vienna, Austria |
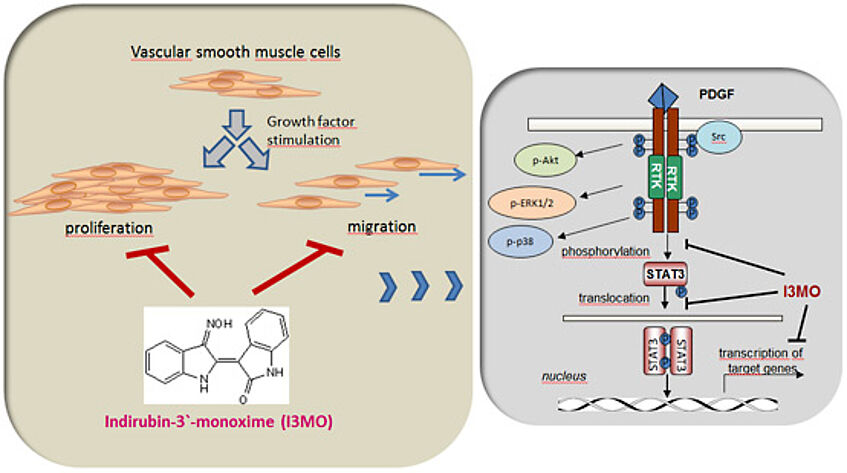
Abnormal proliferation and migration of vascular smooth muscle cells (VSMC) play an important role in the development of cardiovascular disease.
Our previous work showed that indirubin-3-monoxime, a derivative of the red isomer of indigo, exerts potent antiproliferative and antimigratory activity in platelet derived growth factor (PDGF)-stimulated VSMC. I3MO selectively interferes with STAT3 phosphorylation and signalling in VSMC and is able to reduce neointima formation in an arterial cuff model in vivo.We therefore hypothesize that
(i) STAT3 phosphorylation is a crucial signalling step in VSMC proliferation and migration
(ii) I3MO exerts its anti-mito- and anti-motogenic acitivity by inhibition of STAT3 phosphorylation.
Background
Atherosclerosis, cause for cardio-and cerebrovascular diseases, is a major health problem in sedentary societies. Vascular smooth muscle cells (VSMC) are heavily involved in causing vessel lumen reduction by aberrant proliferation and migration.
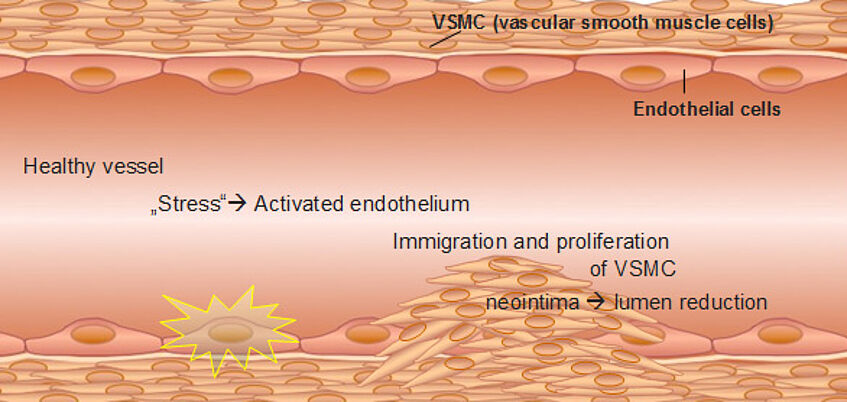
Figure 1: Simplified snapshot of the etiology of atherosclerosis.
In a healthy vessel, vascular smooth muscle cells (VSMC) are responsible for vessel contraction, and endothelial cells (EC), the innermost cells of our blood vessels, maintain vascular homeostasis. If stressed (e.g by high blood pressure or cigarette smoke), the endothelium becomes activated, and the vascular homeostasis collapses. As a result, VSMC immigrate into the vessel lumen and start to divide. A so called neointima is formed leading to lumen reduction as seen in atherosclerosis or restenosis.
Indirubin, the red isomer of blue indigo, is the active constituent of the traditional anti-leukemic Chinese recipe Danggui Langhui Wan.
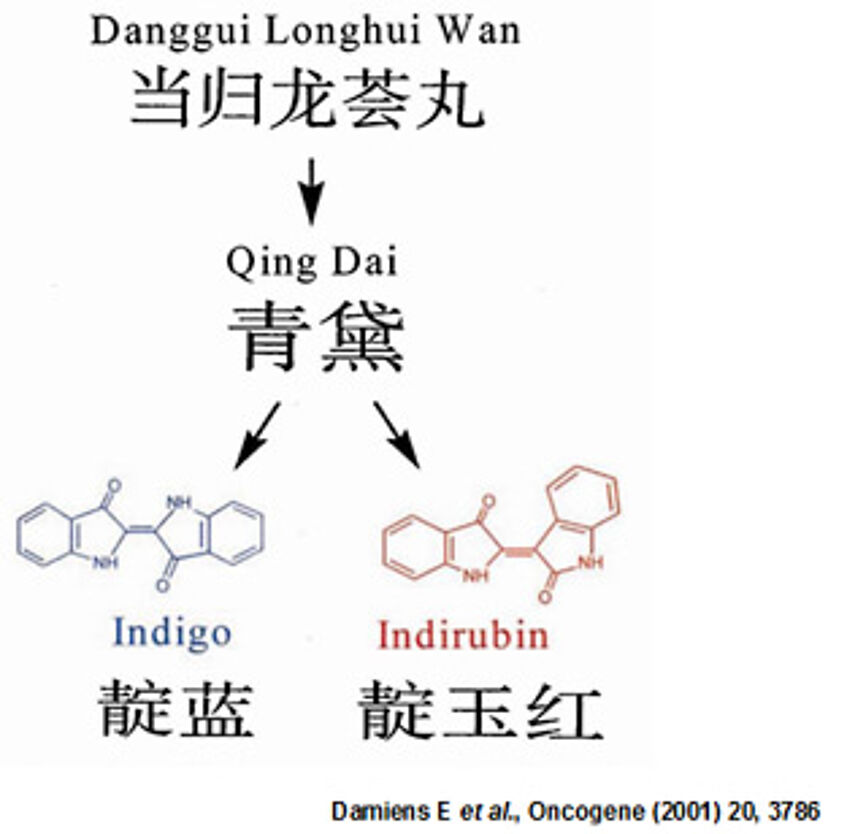
Figure 2: Structure of Indigo and Indirubin
In a previous work we have shown that indirubin-3’-monoxime (I3MO), a more water soluble derivative of indirubin, shows also favourable properties in cell-and animal model of vasculoproliferative diseases, such as atherosclerosis and restenosis. (Arterioscl. Thromb. Vasc. Biol., 2010, 30, 2475).
We demonstrated that I3MO dose-dependently blocks proliferation of growth factor activated vascular smooth muscle cells and inhibits neointima formation in a murine femoral artery cuff model.
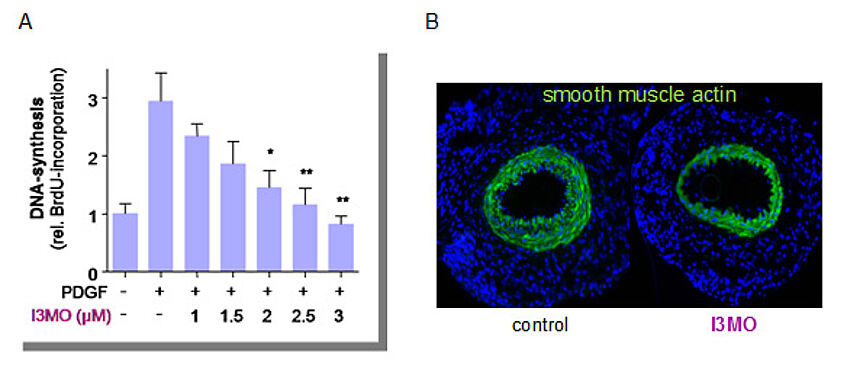
Figure 3: I3MO inhibits VSMC proliferation in vitro and neointima formation in vivo.
(A) Primary rat VSMC were treated with different concentrations of I3MO and stimulated with growth factor (PDGF, platelet derived growth factor) for 4 hrs before DNA replication was assessed via BrdU incorporation.
(B) Neointima formation (as evident by green staining of vascular smooth muscle cells) in femoral arteries cuffed in presence of solvent (control) or I3MO (for methodological details please refer to ATVB, 2010, 30, 2475).
Regarding the mode of action, we revealed that I3MO selectively interferes with phosphorylation and activation of the transcription factor STAT3 (signal transducer and activator of transcription 3) in cultivated and growth-factor stimulated VSMC. Consistently, cuffed arteries show higher levels of active STAT3 in control than in I3MO-treated animals.
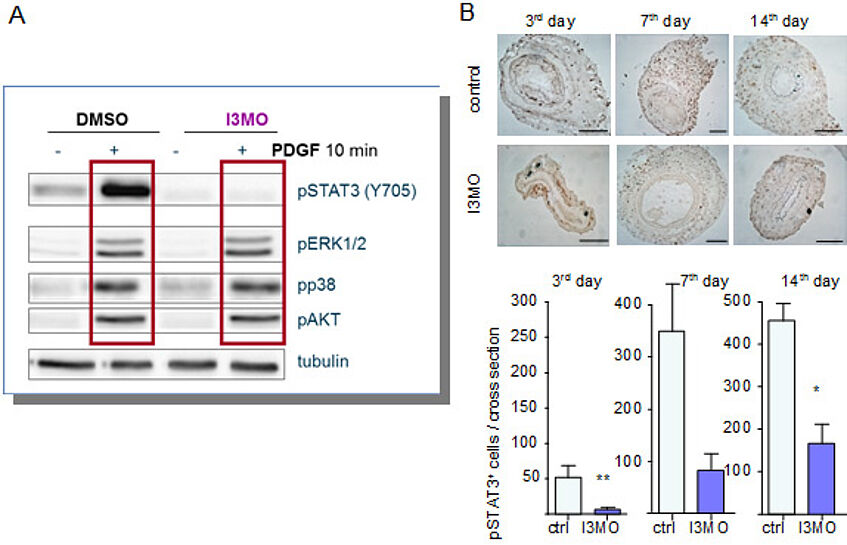
Figure 4: I3MO selectively interferes with STAT3 signalling in activated VSMC and reduces phosphorylated STAT3 in cuffed arteries.
(A) VSMC were treated with I3MO as indicated and stimulated with PDGF before cell lysates were subjected to western blot analysis for the active (=phosphorylated) mitogen activated kinases ERK, p38 and AKT as well as for activated STAT3.
(B) Cuffed arteries were stained for phosphorylated STAT3 (for details please refer to ATVB, 2010, 30, 2475)
Overall, our data highlighted the important role of active STAT3 for the proliferation of vascular smooth muscle cells and the potential of I3MO as lead structure in the battle against vasculoproliferative diseases.
Current Questions
In the ongoing project (started in April 2011) we aim to answer the following main questions:
- What is the detailed molecular mechanism leading to the selective inhibition of STAT3 by I3MO
Special focus is hereby set on kinases, phosphatases and the cellular redox regulation (ROS sources and antioxidant defence proteins) - What is the impact of I3MO and/or STAT3 inhibition on migration of vascular smooth muscle cells, endothelial function and vascular inflammation?
People Involved

Tina Blažević
graduate student
room G2 449
tel +43-1-4277-55239
fax +43-1-4277-55969
e-mail: tina.blazevic@univie.ac.at

Verena Dirsch
co project leader
room 2G 452
tel +43-1-4277-55270
fax +43-1-4277-55969
e-mail: verena.dirsch@univie.ac.at

Elke Heiß
project leader
room 2F 463
tel +43-1-4277-55993
fax +43-1-4277-55969
e-mail: elke.heiss@univie.ac.at
Cooperation Partners
- Univ. Doz. Dr. David Bernhard, Medical University, Vienna, Austria
- Ao Univ. Prof. Johannes Breuss, Medical University, Vienna, Austria
- PD Dr. Robert Fürst, Ludwigs-Maximilian Universität, Munich, Germany
- Univ. Doz. Dr. Mariann Pavone-Gyöngyösi, Medical University, Vienna, Austria
- Prof. Arne Östman, Karolinska Institutet, Stockholm, Sweden
- Prof. Oliver Werz, Friedrich Schiller Universität, Jena, Germany
Related Publications
Original Articles:
Blažević T, Schwaiberger AV, Schreiner CE, Schachner D, Schaible AM, Grojer CG, Atanasov AG, Werz O, Dirsch VM, Heiss EH: 12/15-Lipoxygenase contributes to Platelet-Derived Growth Factor- Induced Activation of Signal Transducer and Activator of Transcription 3. J Biol Chem, 288, 35592-35603 (2013)
more...
Blažević T, Schaible AM, Weinhäupl K, Schachner D, Nikels F, Weinigel C, Barz D, Atanasov AG, Pergola C, Werz O, Dirsch VM, Heiss EH: Indirubin-3’-monoxime exerts a dual mode of inhibition towards leukotriene-mediated vascular smooth muscle cell migration. Cardiovasc Res (2014) in press
more...
Schwaiberger AV, eq Heiss EH,eq Cabaravdic M, Oberan T, Zaujec J, Schachner D, Uhrin P, Atanasov AG, Breuss JM, Binder BR, Dirsch VM. Indirubin-3’-monoxime blocks vascular smooth muscle cell proliferation by inhibition of STAT3 signaling and reduces neointima formation in vivo, Arterioscler Thromb Vasc Biol, 30, 2475-81 (2010)
more...
In Part supported Publications:
Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L, Fakhrudin N, Ladurner A, Malainer C, Vuorinen A, Noha SM, Schwaiger S, Rollinger JM, Schuster D, Stuppner H, Dirsch VM, Heiss EH: Honokiol: a non-adipogenic PPAR gamma agonist from nature. BBA-Gen Sub, 1830, 4813-19 (2013)
Heiss EH, Schachner D, Zimmermann K, Dirsch VM: Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biology, 1, 359-65 (2013)
more...
Kropat C, Mueller D, Boettler U, Zimmermann K, Heiss EH, Dirsch V, Rogoll D, Melcher R, Richling E and Marko D.: In vivo Modulation of Nrf2 Dependent Gene Transcription by Bilberry Anthocyanins – Mediated by the Intestinal Degradation Product Phloroglucinol Aldehyde? Mol Food Nutr Res, 57, 545-50 (2013)
more...
Heiss EH and Dirsch VM: Regulation of eNOS enzyme activity by posttranslational modification. Current Pharmaceutical Design (2014) in press
more...
Heiss EH, Tran TV, Zimmermann K, Schwaiger S, Vouk C, Mayerhofer B, Malainer C, Atanasov AG, Stuppner H, Dirsch VM: Identification of Chromomoric Acid C-I as an Nrf2 Activator in Chromolaena odorata. J Nat Prod (2014) in press
more...
Poster Presentations:
Frühjahrstagung der Deutschen Gesellschaft für Pharmakologie und Toxikologie, April 2011, Frankfurt am Main, Germany
Blazevic T et al.: Inhibition of STAT-3 phosphorylation in vascular smooth muscle cells by indirubin-3’-monoxime is redox-dependent.
59th International Congress and Annual Meeting of the Society for Medicinal Plant and Natural Product Research, September 2011, Antalya, Turkey.
Blazevic T et al.: Is the inhibition of STAT3 phosphorylation by indirubin-3’-monoxime in vascular smooth muscle cells redox-dependent?
3rd EMBO Conference Series : Molecular Medicine and Cellular Signalling, May 2012, Cavtat, Croatia
Blazevic et al.: Inhibition of STAT3 by indirubin-3’-monoxime: investigating the role
of reactive oxygen species.
Interview: Linking Nature with medicine (pdf)
International Innovation is the leading global dissemination
resource for the wider scientific, technology and research communities,
dedicated to disseminating the latest science, research and technological
innovations on a global level.
More information can be found at: www.researchmedia.eu
