FWF P29392 - The role of AMP-activated kinase for Nrf2 mediated stress response
| Project title: | The role of AMP-activated kinase for Nrf2 mediated stress response |
| Project number: | FWF stand alone project P29392 |
| Project leader: | Dr. Elke Heiß elke.heiss@univie.ac.at |
| Duration: | 2016 - 2019 |
| Location: | University of Vienna, Faculty of Life Sciences Department of Pharmacognosy Althanstraße 14, 1090 Vienna, Austria |
Background
AMP-activated kinase (AMPK) is a crucial master hub for controlling cellular energy homeostasis. It is activated when energy gets low, and then enhances processes that provide energy (e.g. respiratory catabolism) and inhibits energy consuming processes (e.g. cholesterol or fatty acid biosynthesis).
Nuclear factor E2 related factor 2 (Nrf2) is a transcription factor that is usually activated when the cell is hit by a harmful insult, such as oxygen radicals or toxins. Upon activation Nrf2 initiates transcription of genes which help the cell to detoxify and get rid of damage, making Nrf2 an important protein to secure cytoprotection and homeostasis.
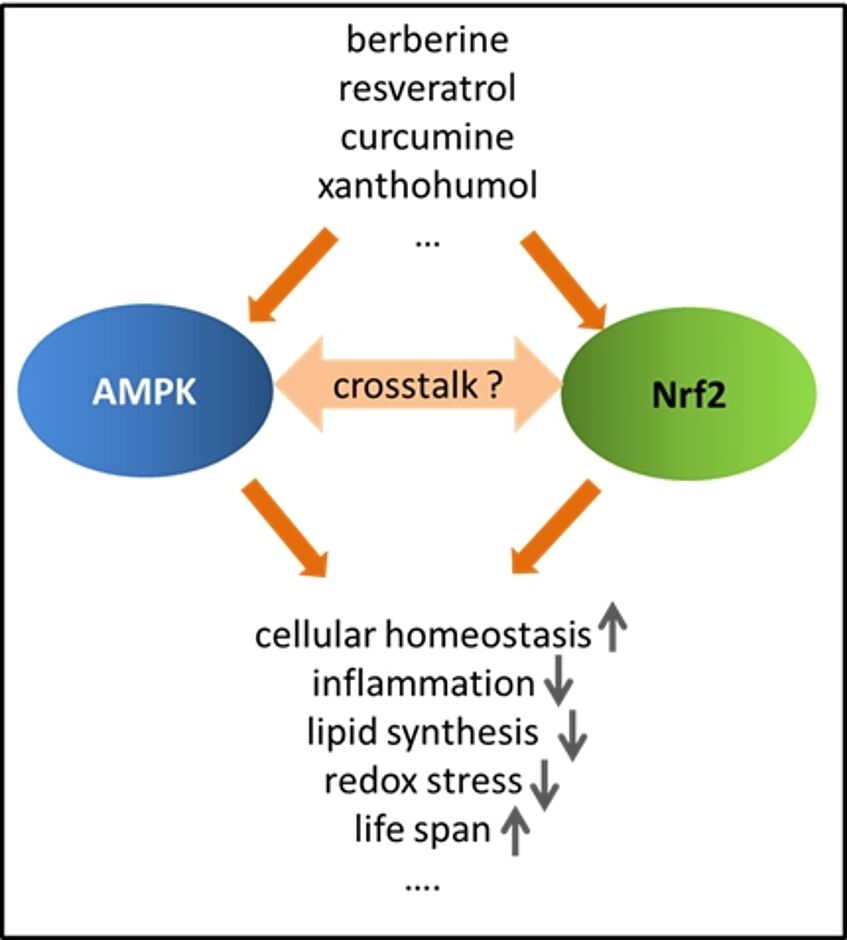
Notably activation of AMPK and Nrf2 signaling elicit overlapping responses in different cellular and animal models, such as reduced inflammation, diminished lipid synthesis, improved glucose homeostasis, elevated stress resistance or even longer lifepan. Moreover, several natural products assumed to exert beneficial health effects lead to activation of both Nrf2 and AMPK signaling (including berberine, resveratrol or curcumine). These observations may suggest a crosstalk between Nrf2 and AMPK. In a previous project we have indeed revealed that AMPK enhances Nrf2-dependent gene transcription of heme oxygenase 1. This and the findings of other groups strongly indicate that the cellular energy state determines via AMPK how and to what extent cells can respond to stressful insults by activation of Nrf2.
References:
Zimmermann K, et al. Activated AMPK boosts the Nrf2/HO-1 signaling axis--A role for the unfolded protein response. Free Radic Biol Med. 2015.
Joo MS, et al. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550.Mol Cell Biol. 2016.
Park JS, et al.. β-Lapachone increases phase II antioxidant enzyme expression via NQO1-AMPK/PI3K-Nrf2/ARE signaling in rat primary astrocytes.Free Radic Biol Med. 2016
Mo C, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014
Hayes JD, Dinkova-Kostova AT: The Nrf2 regulatory network provides an interface between redox and intermediary metabolism.Trends Biochem Sci. 2014
Objectives
In the course of this project we aim at dissecting molecular details of the Nrf2/AMPK crosstalk. We want to address two main questions:
I) Does AMPK lead to posttranscriptional modifications (PTM) of Nrf2, and how do these influence Nrf2 function?
II) Does AMPK exert an influence on the transcription of all Nrf2 target genes or just a subset, and how is this effect accomplished on the molecular level?

The answers to these questions will provide important and novel insight into the incompletely understood crosstalk between cellular energy homeostasis and stress signaling. The findings may also foster the understanding why and how caloric restriction (AMPK activation) on an organismal level accounts for the observed life span prolongation due to increased stress resistance and ultimately offer new inspiration for the development of drugs/nutraceuticals (dual Nrf2 and AMPK activators) aiming for healthy aging.
People Involved

Elke Heiß
project leader
room 2F 463
tel +43-1-4277-55993
fax +43-1-4277-55969
e-mail: elke.heiss@univie.ac.at

Manuel Matzinger
graduate student
room G2 407
tel +43-1-4277-55225
fax +43-1-4277-55969
e-mail: manuel.matzinger@univie.ac.at

Katrin Fischhuber
graduate student
room G2 407
tel +43-1-4277-55225
fax +43-1-4277-55969
e-mail: katrin.fischhuber@univie.ac.at

Karl Mechtler
Co-PI (MS-based proteomics), IMBA/IMP

Manfred Ogris
Co-PI (in vivo Imaging), MMCT Lab, University of Vienna
